Whilst chimeric antigen receptor (CAR) T cells have shown remarkable success, further novel targets are required. However, our previous cell surface proteomics work in multiple myeloma (MM) showed that target choice is highly limited by an absence of truly tumour-specific antigens, and often very low on-tumour expression of target proteins in some primary samples. One approach to improve tumour specificity is to use combinatorial antigen recognition, or “AND-gates”, whereby a CAR T cell must recognise two different antigens for full activation and target cell elimination. It has also been reported that this approach might overcome low target expression. We therefore decided to explore the potential for AND-gate targeting in MM and identify a suitable target pairing.
To determine a comprehensive list of viable AND-gate combinations for MM, we first integrated our MM proteomics data with a normal tissue proteomics dataset. From 777 extracellular proteins expressed in primary MM, we identified a total of 287,890 possible pairwise combinations. We next excluded any pairs with overlapping expression in vital, healthy tissues, reducing potential combinations to 664. Additional filtering steps to remove difficult-to-target proteins and combinations where either target was expressed on T cells - thus avoiding T cell fratricide - resulted in a final list of 92 AND-gate targets. From our prioritised candidates, we selected TNFRSF8 and TMPRSS11E as an exemplar pairing. By mass spectrometry, both TNFRSF8 and TMPRSS11E were expressed across all 8 profiled primary MM samples at comparable levels to existing CAR T cell targets, such as GPRC5D, and had no predicted overlapping expression in normal tissue. To test our AND-gate combination, we designed a ‘split-CAR’ system in which TNFRSF8 was targeted by a 1 st generation CAR with an intracellular signalling domain, but without a co-stimulatory receptor (CAR-only construct), and TMPRSS11E was targeted by a construct with a chimeric co-stimulatory receptor (CCR), but lacking an intracellular signalling domain (CCR-only construct).
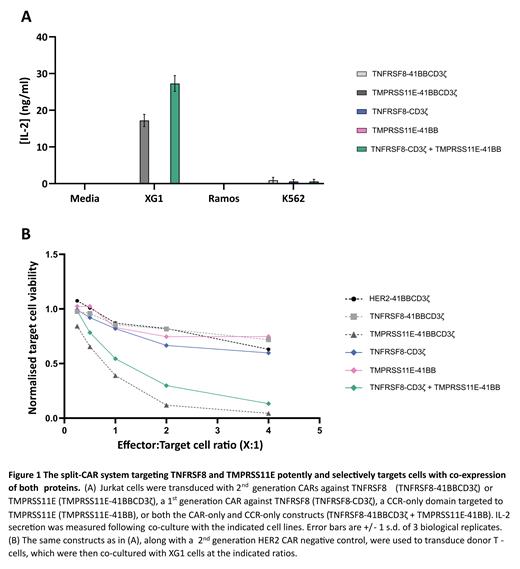
Constructs were initially validated in a Jurkat cell line model of T cell activation. Jurkats were transformed with CCR-only, CAR-only, or both constructs, as well as with full 2 nd generation CARs targeting TNFRSF8 or TMPRSS11E. These cells were co-cultured with the TNFRSF8+TMPRSS11E+ human MM cell line XG1 or the TNFRSF8+TMPRSS11E- erythroleukemic cell line K562. As anticipated, neither the CCR-only nor the CAR-only Jurkats were activated, with no production of IFNγ or IL2 ( Fig. 1A). Whilst Jurkats expressing the full 2 nd generation TMPRSS11E CAR were activated in co-culture with XG1, Jurkats expressing the TNFRSF8 2 nd generation CAR were not, despite expression of TNFRSF8 on XG1. Jurkats co-expressing both components of the split-CAR were markedly activated when co-cultured with TNFRSF8+TMPRSS11E+ XG1 cells (but not with TNFRSF8+TMPRSS11E- K562 cells), as demonstrated by a large increase in expression of CD69, IFNγ, and IL2 ( Fig. 1A). Thus, the split-CAR system showed a very high degree of specificity, requiring both antigens to be co-expressed on the target cell. Moreover, it was activated even when full 2 nd generation CARs against TNFRSF8 were not.
Next, we sought to investigate the cytolytic activity of our split-CAR constructs in donor T cells. In a cytotoxicity assay, T cells expressing the CAR-only or CCR-only construct did not exhibit significant killing of XG1 cells ( Fig. 1B). In parallel with the Jurkat reporter assays, only 2 nd generation TMPRSS11E CAR T cells, and not 2 nd generation TNFRSF8 CAR T cells, were cytotoxic to XG1. Finally, donor T cells expressing both components of the split-CAR demonstrated potent killing of dual-positive XG1 cells ( Fig. 1B), but not single-positive K562 cells.
We have thus developed an analytical pipeline for the discovery of AND-gate CAR T cell targets for MM, also applicable to other tumours. This approach led to the development of an extremely potent, but highly selective, split-CAR targeting TNFRSF8 and TMPRSS11E. Importantly, our split-CAR targeted very low levels of TNFRSF8 antigen that were insufficient to trigger activation of a 2 nd generation TNFRSF8 CAR-T. In summary, our work suggests that AND-gate targeting can increase not just specificity, but potency, even against very low expression tumour targets in MM.
Link to preprint https://doi.org/10.1101/2023.04.04.535580
Disclosures
Walker:Abbvie: Honoraria; Janssen: Honoraria. Chapman:Celgene (BMS): Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal